


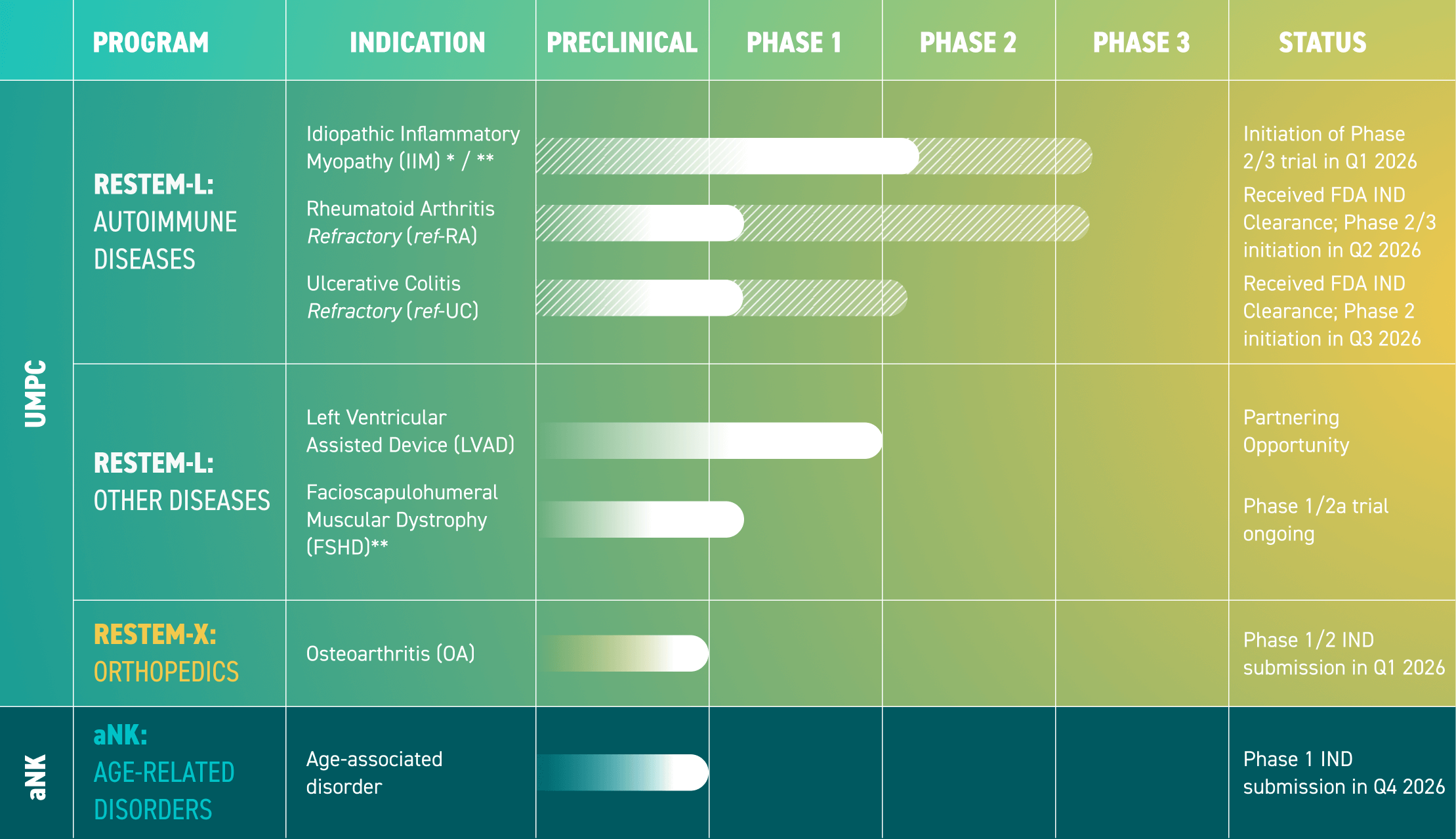

RESTEM’s investigational UMPC therapy, Restem-L, has shown incredible promise in potency, safety, and efficacy across numerous for patients with autoimmune diseases. In preclinical and clinical studies, it was shown that Restem-L has a superior immunomodulatory profile compared to other types of mesenchymal stem cells (MSCs) such as bone marrow or fat MSCs.
In a Phase1 clinical trial in idiopathic inflammatory myopathy, Restem-L was well tolerated and led to clinically meaningful and durable improvements in the total improvement score (TIS) of patients. In addition, all patients experienced significant improvements in manual muscle testing and extra muscular disease sustained throughout the study. Notably, treatment with Restem-L resulted in a significant reduction in steroid dosage of patients within 6 months, indicating its potential to modulate inflammation in the immune system.
In a Phase1 clinical trial in left ventricular assist device (LVAD) patients with bleeding complications, Restem-L was noted to be safe and tolerable and did not cause immune sensitization. Half of the patients were noted to have angiogenic stabilization, and there was a trend toward decreasing amounts of blood noted in the stool, suggesting an early signal of efficacy.
RESTEM has dedicated years to aNK cell research with the goal of restoring the immune system to a younger, more active state. RESTEM has developed a novel, patient-specific personalized approach to produce highly activated and ex-vivo expanded, clinical grade aNK cells in a cost-effective, GMP-compliant manner. These aNK cells can also be administered without immunosuppressive preconditioning, potentially improving the effectiveness of the therapy.
In a small pilot study assessing immunosenescence in patients, data suggests that one or two infusions of aNK cells are safe and may reduce immunosenescence for up to six months. This represents a new therapeutic approach to reduce the detrimental features of aging such as inflammation and immunosenescence.